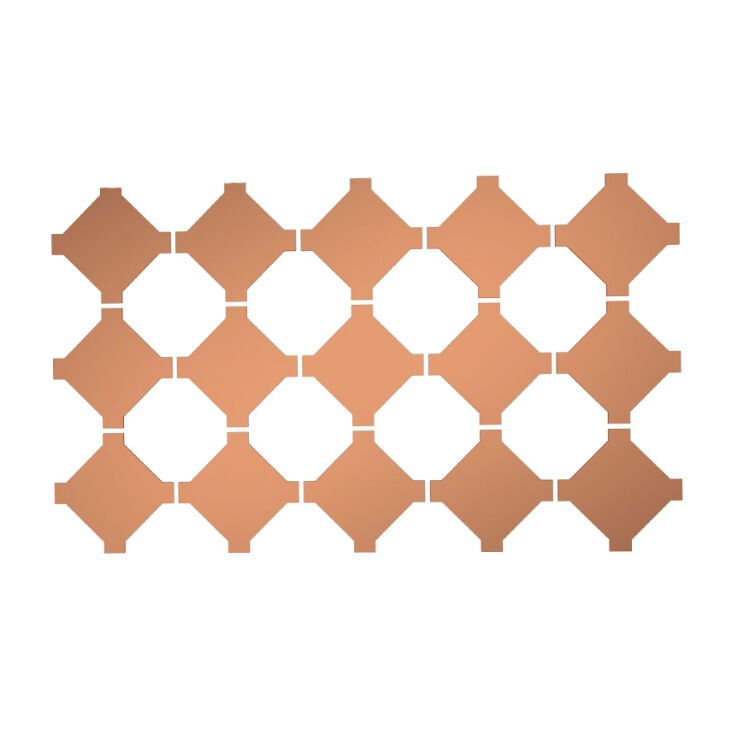
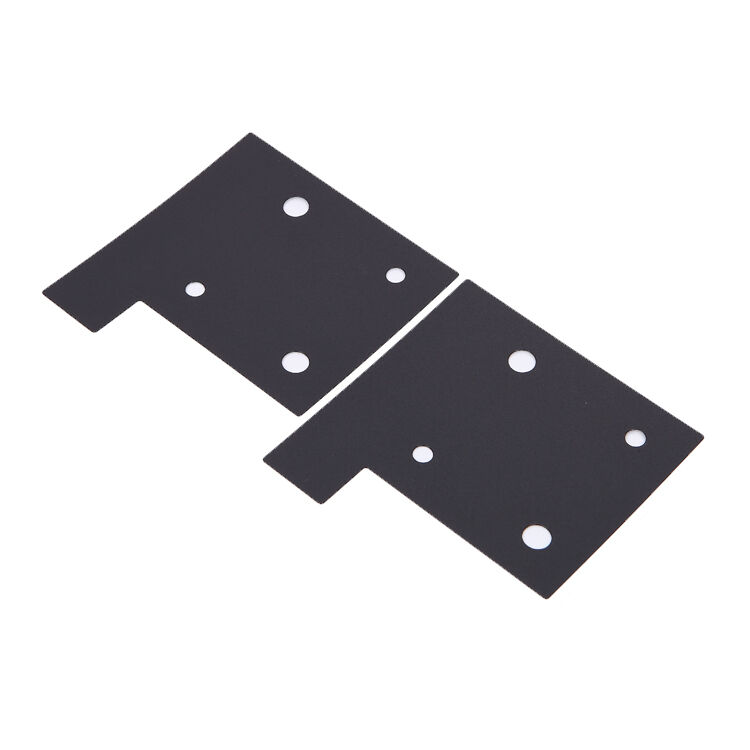
Introduction
In the rapidly evolving landscape of medical electronics, the demand for specialized conductive materials that meet stringent regulatory standards has never been more critical. Healthcare manufacturers and electronic device integrators worldwide are increasingly seeking reliable solutions that combine exceptional electrical performance with complete environmental compliance. The RoHS REACH Compliant Conductive Sponge for Medical Electronics represents a breakthrough in advanced material engineering, specifically designed to address the complex requirements of modern medical device applications while ensuring full adherence to international safety and environmental regulations.
Medical electronics applications require materials that not only deliver consistent electrical conductivity but also maintain their performance characteristics under demanding conditions typical of healthcare environments. Traditional conductive materials often fall short when subjected to the rigorous testing protocols mandated by medical device standards, leading to compromised device reliability and potential regulatory issues. This innovative conductive sponge technology bridges the gap between superior electrical performance and regulatory compliance, offering medical device manufacturers a dependable solution for their most critical applications.
Product Overview
The RoHS REACH Compliant Conductive Sponge for Medical Electronics is engineered using advanced polymer matrix technology infused with carefully selected conductive particles that maintain stable electrical properties across varying environmental conditions. This specialized foam material combines the flexibility and compressibility of traditional sponge materials with the electrical conductivity required for sensitive medical electronic applications. The unique cellular structure provides optimal balance between mechanical resilience and electrical performance, making it ideally suited for applications requiring both cushioning and electrical connectivity.
Manufactured through a proprietary process that ensures uniform distribution of conductive elements throughout the foam matrix, this material delivers consistent electrical characteristics across the entire surface area. The foam's open-cell structure allows for excellent conformability while maintaining reliable electrical pathways, even under repeated compression cycles. This design philosophy ensures that medical devices incorporating this conductive sponge maintain their electrical integrity throughout their operational lifespan, contributing to enhanced device reliability and patient safety.
Features & Benefits
Superior Electrical Performance
The conductive sponge exhibits exceptional electrical stability across a wide range of operating conditions, making it particularly valuable for medical electronics that must function reliably in various healthcare environments. The material's resistance characteristics remain consistent even when subjected to temperature fluctuations, humidity variations, and mechanical stress typical of medical device applications. This stability is crucial for maintaining signal integrity in sensitive medical monitoring equipment and diagnostic devices.
Enhanced Mechanical Properties
Beyond its electrical capabilities, this RoHS REACH Compliant Conductive Sponge for Medical Electronics offers superior mechanical performance characteristics that make it ideal for demanding medical applications. The foam maintains its structural integrity under repeated compression and decompression cycles, ensuring long-term reliability in applications where mechanical stress is a constant factor. The material's resilience and recovery properties contribute to consistent electrical contact over extended periods of use.
Environmental Compliance
Complete adherence to both RoHS and REACH regulations ensures that this conductive material meets the most stringent international environmental standards without compromising performance. The formulation excludes hazardous substances while maintaining the electrical and mechanical properties required for critical medical applications. This compliance framework provides manufacturers with confidence that their end products will meet global regulatory requirements for medical device manufacturing.
Applications & Use Cases
The versatility of this RoHS REACH Compliant Conductive Sponge for Medical Electronics makes it suitable for a diverse range of medical device applications where both electrical conductivity and cushioning properties are essential. In medical imaging equipment, the material serves as an effective electromagnetic shielding component while providing vibration dampening for sensitive electronic assemblies. The foam's ability to conform to irregular surfaces makes it particularly valuable in applications where traditional rigid conductors cannot provide adequate coverage or protection.
Patient monitoring systems benefit significantly from the material's stable electrical properties and biocompatible formulation. The conductive sponge can be integrated into electrode assemblies, sensor housings, and cable management systems where electrical continuity must be maintained while accommodating patient movement and device positioning adjustments. Its soft, compressible nature enhances patient comfort while ensuring reliable electrical connections throughout extended monitoring periods.
Diagnostic equipment manufacturers utilize this conductive foam in applications requiring electromagnetic interference shielding combined with shock absorption capabilities. The material's cellular structure provides effective attenuation of unwanted electromagnetic signals while protecting delicate electronic components from mechanical vibrations and impacts. This dual functionality is particularly valuable in portable diagnostic devices that must operate reliably in challenging clinical environments.
Quality Control & Compliance
Manufacturing processes for this RoHS REACH Compliant Conductive Sponge for Medical Electronics incorporate comprehensive quality control measures designed to ensure consistent product performance and regulatory compliance. Every production batch undergoes rigorous testing protocols that verify electrical properties, mechanical characteristics, and environmental compliance parameters. These quality assurance procedures guarantee that each shipment meets the exacting standards required for medical device applications.
The material's compliance with international medical device standards extends beyond basic RoHS and REACH requirements to encompass biocompatibility testing and long-term stability assessments. Extensive validation testing ensures that the conductive sponge maintains its performance characteristics throughout typical medical device lifecycles while remaining safe for use in healthcare environments. Documentation packages accompanying each shipment provide comprehensive compliance certificates and test reports required for medical device manufacturer quality systems.
Continuous monitoring of raw material sources and manufacturing processes ensures ongoing compliance with evolving regulatory requirements. The quality management system incorporates feedback mechanisms that allow for rapid response to changing industry standards and customer requirements. This proactive approach to quality control helps medical device manufacturers maintain their own regulatory compliance while benefiting from consistent material performance.
Customization & Branding Options
Recognizing that medical device applications often require specialized material configurations, extensive customization options are available for this RoHS REACH Compliant Conductive Sponge for Medical Electronics. Custom cutting and shaping services allow manufacturers to obtain precisely configured components that integrate seamlessly into their device designs. Die-cutting capabilities accommodate complex geometries and tight tolerances required for medical device assemblies.
Surface treatment options enhance the material's compatibility with various assembly processes and environmental conditions. Adhesive backing applications, surface coatings, and lamination services provide additional functionality while maintaining the material's core electrical and mechanical properties. These customization options enable medical device manufacturers to optimize their assembly processes and improve overall product reliability.
Private labeling and custom packaging services support manufacturer branding requirements while ensuring proper material identification and traceability throughout the supply chain. Custom documentation packages can include specific test reports, compliance certificates, and technical specifications tailored to individual customer requirements. This level of customization support helps medical device manufacturers maintain their quality systems while benefiting from specialized material expertise.
Packaging & Logistics Support
Specialized packaging solutions protect the integrity of this RoHS REACH Compliant Conductive Sponge for Medical Electronics throughout transportation and storage phases. Anti-static packaging materials prevent electrical damage while moisture barrier properties maintain material performance characteristics during extended storage periods. Custom packaging configurations accommodate various order quantities while optimizing shipping efficiency and material protection.
Global logistics capabilities ensure reliable delivery to medical device manufacturers worldwide while maintaining cold chain integrity where required. Comprehensive tracking systems provide real-time visibility into shipment status and delivery schedules, enabling manufacturers to optimize their production planning and inventory management processes. Flexible shipping options accommodate both standard delivery schedules and expedited requirements for urgent medical device production needs.
Inventory management services help medical device manufacturers optimize their material procurement strategies while ensuring consistent supply availability. Just-in-time delivery options reduce inventory carrying costs while maintaining production schedule reliability. These logistics support services enable manufacturers to focus on their core competencies while benefiting from specialized material supply chain expertise.
Why Choose Us
With extensive experience serving international medical device markets across multiple continents, our organization has developed deep expertise in advanced conductive materials and regulatory compliance requirements. Our global presence enables us to provide consistent support to medical device manufacturers regardless of their geographic location, while our multi-industry experience brings valuable insights from aerospace, telecommunications, and industrial electronics applications to medical device challenges.
As a recognized conductive material specialist, we maintain strategic partnerships with leading research institutions and regulatory bodies to ensure our products remain at the forefront of technological advancement and compliance requirements. Our engineering team collaborates closely with medical device manufacturers to develop customized solutions that address specific application challenges while maintaining full regulatory compliance. This collaborative approach has resulted in successful implementations across diverse medical device categories and global markets.
Our commitment to quality excellence and customer satisfaction has established long-term relationships with medical device manufacturers who rely on our expertise for their most critical applications. Continuous investment in manufacturing capabilities and quality systems ensures that we can meet evolving customer requirements while maintaining the highest standards of product consistency and reliability. This dedication to excellence has positioned us as a trusted partner for medical device manufacturers seeking dependable conductive material solutions.
Conclusion
The RoHS REACH Compliant Conductive Sponge for Medical Electronics represents a significant advancement in specialized materials for medical device applications, combining exceptional electrical performance with complete environmental compliance in a single, versatile solution. Its unique combination of conductivity, mechanical resilience, and regulatory adherence makes it an ideal choice for medical device manufacturers seeking to enhance their product reliability while maintaining full compliance with international standards. The material's proven performance across diverse medical applications, coupled with comprehensive customization and support services, provides manufacturers with a dependable foundation for their most demanding electrical connectivity requirements. As medical device technology continues to evolve, this innovative conductive sponge solution offers the performance consistency and regulatory compliance necessary to support the next generation of healthcare innovations.
Professional EMI Shielding Tapes - Total Solution for RFI/ESD Protection
Excellent Shielding Performance |
Offers high shielding effectiveness over a wide frequency range, ideal for EMI, RFI, and ESD full protection. |
||||||
High Conductivity & Low Resistance |
Made from superior materials like nickel/copper coated fabric or pure copper foil, ensuring good electrical conductivity and reliable grounding. |
||||||
Flexible & Conformable |
Ultra-thin, flexible, and lightweight design allows for easy application on curved surfaces and tight spaces, such as internal circuit boards and flexible circuits. |
||||||
Customizable Solutions |
Available in custom sizes, shapes, and die-cuts (OEM/ODM supported). RoHS and REACH compliant to meet global environmental standards. |
||||||
Consumer Electronics |
Smartphones, laptops, gaming consoles, LCD displays, and 5G wireless chargers for internal circuit board shielding and high-speed HDMI signal protection. |
||||||
Automotive & Transportation |
Automotive electronics, autonomous vehicles LiDAR/Radar systems, and EV wireless chargers as part of robust EMC solutions. |
||||||
Telecom & Networking |
Networking equipment, 5G infrastructure, and cabinet shielding to ensure signal integrity and prevent interference. |
||||||
Industrial & Medical
|
Industrial control systems, power supplies, and sensitive medical electronic devices requiring reliable performance in demanding conditions. |
||||||
Available Types
Company Profile
We cater primarily to the consumer electronics, communication networks, and emerging energy vehicle sectors. To back ourcommitment to quality and innovation, we have established a national production base across four strategic locations:
1. A 1,000-square-meter new material formula R&D center in Shenzhen.
2. A 2,000-square-meter innovative grounding wrapping production plant in Dongguan.
3. A 10,000-square-meter conductive shielding tape coating plant in Hunan.
4. A 1,000-square-meter magnetron sputtering continuous gold/tin plating plant in Shandong.
As the only vertically integrated integrator in China offering electroplating, coating, and processing capabilities, we strive to become a leading innovator and provider of core values within the materials industry chain. Our ultimate goal is to be a long-term trusted solution provider and partner for our customers in addressing their challenges.
Invention Patent




System certification






FAQ
We are based in Guangdong, China, start from 2011,sell to South Asia(10.00%). There are total about 101-200 people in our office.
2. how can we guarantee quality?
Always a pre-production sample before mass production;
Always final Inspection before shipment;
3.what can you buy from us?
Shielding tape,Adhesive tapes,Waterproof and breathable membrane,Customized production,Raw material die-cutting
4. why should you buy from us not from other suppliers?
Shenzhen Johan Material Tech Co., Ltd. was established in 2011, and is an innovative national high-tech enterprise providing
innovative EMC (electromagnetic compatibility) grounding elastomers and customized tape solutions for the electronics indus
5. what services can we provide?
Accepted Delivery Terms: FOB,CFR,CIF,EXW,FAS,CIP,FCA,CPT,DEQ,DDP,DDU,Express Delivery,DAF,DES;
Accepted Payment Currency: USD,EUR,JPY,CAD,AUD,HKD,GBP,CNY,CHF;
Accepted Payment Type: T/T,L/C,D/P D/A,MoneyGram,Credit Card,PayPal,Western Union,Cash,Escrow;
Language Spoken: English,Chinese